CSP is entitled to carry out manufacturing operations limited to secondary pharmaceutical products packaging.
Dedicated manufacturing operations locations enable the upmost care in the handling of products and guarantee perfect control at each step of the manufacturing procedure in accordance with Good Manufacturing Practices.
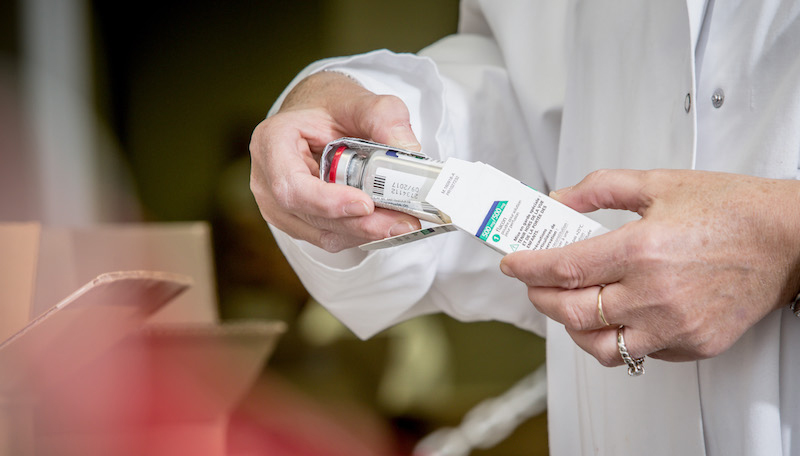
Prior to validating the batch by the laboratory and after the completing of the batch file a specially trained team carries out every operation paying strict attention to achieving the indispensable controls and the follow up quality process. The files thus documented are systematically validated and endorsed by the pharmacist.
The manufacturing service from CSP perform manual operations (bumper stickers, labelling, peal-off vignettes and packaging,…) so as to have clean and well cared for results and in line with the catalogue of requirements laid down by the laboratories : changes in the instruction for use leaflet
Any other service provision can be studied on request.
In addition, our manufacturing department proposes packaging checks, datamatrix checking, all processes are under CSP Pharmacist control.
Finally, their manufacturing service is able to realize on request kits and promotional packs, operating of packing changes or even the fashioning of display racks.
Technological Innovation at the service of healthcare:

CSP has been progressing throughout the years to meet all the needs of their customers and invest themselves in the use of the upmost advanced security and identification technologies in achieving this goal. Accordingly, they already propose to their customers the use of innovative reading technologies and Data matrix 2D marking technology.
CSP is qualified as a human and veterinary manufacturing pharmaceutical business.

