The aim of serialisation is to affix a unique identifier to each box of medicinal products. This obligation aims to reinforce the identification of each product unit so as to combat counterfeiting and reduce public health risks related to fraud as much as possible.
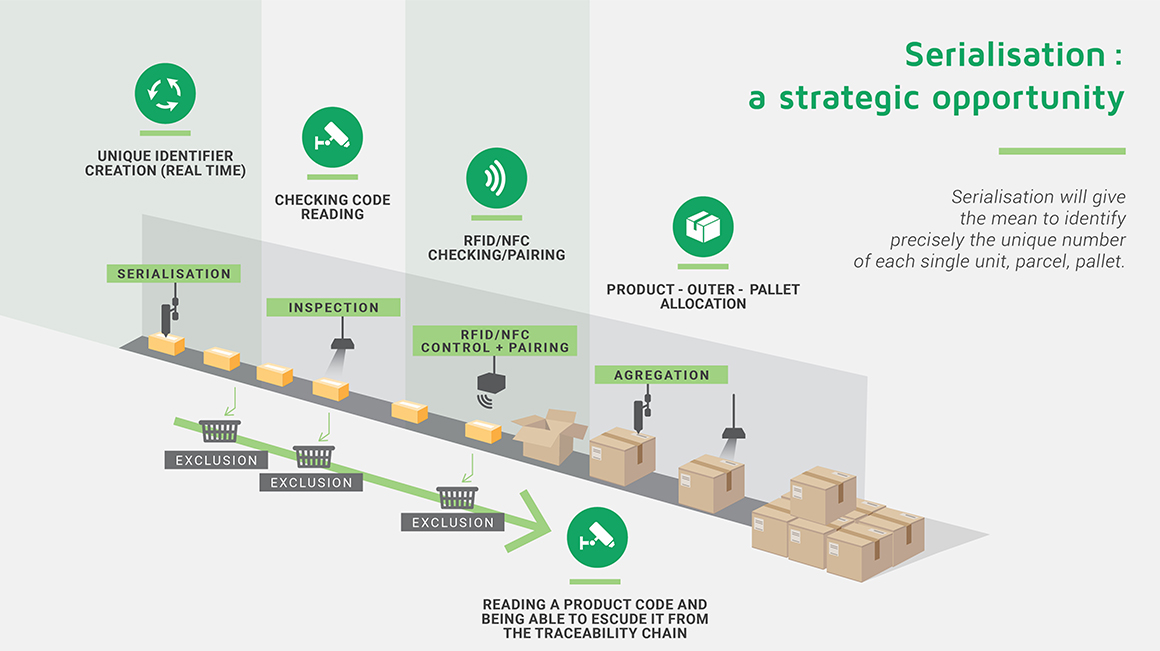
CSP offers a range of flexible services. Capable of generating unique numbers for boxes of medicinal products in compliance with specific encoding algorithms, Digital CSP can take into account the specifications of every sub-contracting toller and adapt data interchange with the appropriate safety and level of encoding. CSP acts as a trusted intermediary between the manufacturers, their pharmaceutical laboratory contract givers and the centralising authorities for serialisation.

CSP ensures the control of serialized units by analysing the Data Matrix, identifying serial numbers, checking of the presence in national databases, as well as the presence of tamper-proof devices. Depending on the situation, CSP carries out decommissioning operations and in some cases may perform exceptional recommissioning operations. The complete traceability of operations gives rise to a detailed retrieval of the lists of control units, decommissioned per type of movement and pharmaceutical status.
CSP has also invested in a serialization unit to handle the printing of serial numbers on boxes of medicinal products. This integrated solution follows the rules of Good Manufacturing Practices and makes it possible to meet the small run production needs of laboratories.
